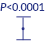Menu
ABOUT MOVENTIG/SBM EFFICACY
Address the hidden burden of OIC within hours
Study design: Data were pooled from 2 identical, 12-week, multicentre, randomised, double-blind, placebo-controlled, phase 3 trials of naloxegol in patients with non-cancer pain, OIC, and an inadequate response to laxatives in the United States and Europe (KODIAC-04 and KODIAC-05). Patients received naloxegol 12.5mg (n=240), naloxegol 25mg (n=241), or placebo (n=239). The primary endpoint, assessed in the ITT population, was response during the 12-week treatment period, defined as having ≥3 spontaneous bowel movements (SBM) per week and an increase from baseline of ≥1 SBM per week, for ≥3 of the final 4 weeks.
Efficacy of MOVENTIG in treating OIC*1,2
Less than 8 hours median time to first SBM with MOVENTIG1,2
41.1
7.6
- MOVENTIG demonstrated a significantly shorter median time to first spontaneous bowel
movement (SBM) vs placebo (P<0.001)1,2 - 67% of patients taking MOVENTIG 25mg had their first bowel movement within 24 hours
of the first dose1 - The responder rates in this population were placebo, 30.0%; MOVENTIG 12.5mg, 44.3%,
and MOVENTIG 25mg, 44.4%1,2
MOVENTIG also provided meaningful benefits in
other bothersome symptoms of OIC
other bothersome symptoms of OIC
Patients reported significant improvement in straining, stool consistency, and
completeness of bowel movements compared with placebo over weeks 1 to 12.1
completeness of bowel movements compared with placebo over weeks 1 to 12.1
%
improvement in
stool consistency1
stool consistency1
LS mean (SEM): naloxegol 25mg (n=238),
0.70, (0.06); placebo (n=238), 0.40
0.70, (0.06); placebo (n=238), 0.40
%
reduction
in straining1
in straining1
LS mean (SEM): naloxegol 25mg (n=238),
-0.77, (0.06); placebo (n=238), -0.49
-0.77, (0.06); placebo (n=238), -0.49
*MOVENTIG is indicated for the treatment of opioid-induced constipation (OIC) in adult patients who have had an inadequate response to laxative(s). To qualify as a laxative-inadequate responder, in the 2 weeks prior to the first study visit patients had to have reported concurrent OIC symptoms of at least moderate severity while taking at least 1 laxative class for a minimum of 4 days during the pre-study period.
OIC, opioid-induced constipation; LS mean, least squares mean.
Before prescribing MOVENTIG, please review the Summary of Product Characteristics.
Treatment with MOVENTIG 25mg led to clinically relevant and statistically significant improvements in QOL scores that were sustained for 12 months (P<0.0001)3
Study design: One-year prospective, open-label, real-world study of patients with cancer, with a confirmed diagnosis of OIC with LIR, conducted in 12 Spanish provinces. The primary endpoint was the assessment of constipation-related QOL in patients who received MOVENTIG using the PAC-QOL. Secondary endpoints included evaluation of the efficacy of MOVENTIG in treating OIC over follow-up. Of 126 patients, 85 completed 3 months of follow-up, 55 completed 6 months, and 29 completed 12 months.3
OIC, opioid‐induced constipation; PAC‐QOL, Patient Assessment of Constipation Quality of Life Questionnaire; QOL, quality of life.
Consider the clinical safety of MOVENTIG.
Learn More 
Reference 1. Tack J, Lappalainen J, Diva U, Tummala R, Sostek M. Efficacy and safety of naloxegol in patients with opioid–induced constipation and laxative–inadequate response. United Eur Gastroenterol J. 2015;3(5):471–480. doi:10.1177/2050640615604543 2. Tack J, Lappalainen J, Diva U, Tummala R, Sostek M. Efficacy and safety of naloxegol in patients with opioid–induced constipation and laxative–inadequate response. Supplementary tables. United Eur Gastroenterol J. 2015;3(5):471–480. doi:10.1177/2050640615604543 3. Cobo Dols M, Zambrano CB, Cabezón–Gutiérrez L, et al. One–year efficacy and safety of naloxegol on symptoms and quality of life related to opioid–induced constipation in patients with cancer: KYONAL study. BMJ Support Palliat Care. 2021;0:1–9. doi:10.1136/bmjspcare–2020–002816
VIEW PRESCRIBING INFORMATION 

PRESCRIBING INFORMATION (prepared August 2021)
Moventig® (naloxegol oxalate) 12.5mg and 25mg film-coated tablets
Consult Summary of Product Characteristics (SmPC) before prescribing.
Indication: Opioid-induced constipation (OIC) in adult patients who have had an inadequate response to laxative(s) (concurrent OIC symptoms of at least moderate severity while taking at least one laxative class for a minimum of four days during the previous 2 weeks).
Dosage and administration: Recommended 25 mg once daily. Take on empty stomach at least 30 minutes prior to first meal of day or 2 hours after first meal of day. Crushed tablets can be mixed with water (120ml) and drunk immediately or administered via a nasogastric tube (CH8 or greater). Renal impairment: Moderate or severe renal impairment starting dose 12.5mg. Discontinue if side effects impact tolerability. Increase to 25mg if well tolerated. Hepatic impairment: Use in severe hepatic impairment not recommended. Moderate CYP3A4 inhibitors: Starting dose 12.5mg, can be increased to 25mg if well tolerated. Paediatric population (<18 years): Safety and efficacy not yet established.
Adverse effects: Consult SmPC for full list of side effects. Very Common: Abdominal pain, diarrhoea. Common: Nasopharyngitis, headache, flatulence, nausea, vomiting, hyperhidrosis. Uncommon: Opioid withdrawal syndrome. Not known: Hypersensitivity, Gastrointestinalperforation.
Contraindications: Hypersensitivity to active substance or any of the excipients or any other opioid antagonist. Patients with known or suspected gastrointestinal (GI) obstruction or patients at increased risk of recurrent obstruction. Patients with underlying cancer who are at heightened risk of GI perforation, such as those with underlying malignancies of gastrointestinal tract or peritoneum, recurrent or advanced ovarian cancer or vascular endothelial growth factor (VEGF) inhibitor treatment. Concomitant use with strong CYP3A4 inhibitors.
Warnings and precautions: Cases of gastrointestinal perforation have been reported in the post-marketing setting, including fatal cases when naloxegol was used in patients who were at an increased risk of gastrointestinal (GI) perforation. Naloxegol must not be used in patients with known or suspected gastrointestinal obstruction or in patients at increased risk of recurrent obstruction. Use with caution in patients with any condition which might result in impaired integrity of the gastrointestinal tract wall. Advise patients to discontinue therapy and promptly report if unusually severe or persistent abdominal pain develops. Use with caution in patients with clinically important disruptions to the blood brain barrier and observe for potential CNS effects. Discontinue if interference with opioid-mediated analgesia or opioid withdrawal syndrome occurs. Use with caution in patients taking methadone. If opioid withdrawal syndrome is suspected the patient should discontinue Moventig and contact their physician. Use with caution in patients with a recent history of myocardial infarction, symptomatic congestive heart failure, overt cardiovascular (CV) disease or with a QT interval of ≥500msec. Use with caution in OIC patients with cancer-related pain. Use of naloxegol with another opioid antagonist (e.g. naltrexone, naloxone) should be avoided.
Use in pregnancy and lactation: Not recommended.
Legal category: POM.
Marketing Authorisation numbers: Moventig 12.5mg and 25mg tablets (ROI: EU/1/14/962/001-011),(GB: PL GB 50262/004&5)
Further information available on request from the Marketing Authorisation holder: Kyowa Kirin Holdings B.V., Bloemlaan 2, 2132NP Hoofddorp, The Netherlands.
For the United Kingdom:
NHS cost: Moventig 12.5mg, 30 tablets, £55.20; Moventig 25mg, 30 tablets, £55.20.
NHS cost: Moventig 12.5mg, 30 tablets, £55.20; Moventig 25mg, 30 tablets, £55.20.
Adverse Events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse Events should also be reported to Kyowa Kirin Ltd. on +44 (0)1896 664000, email medinfo@kyowakirin.com
For the Republic of Ireland:
Adverse Events should be reported. Information about adverse event reporting can be found at www.hpra.ie. Adverse Events should also be reported to Kyowa Kirin Ltd. on +44 (0)1896 664000, email medinfo@kyowakirin.com
This program is best viewed on a device with a higher screen resolution. Some content may not be optimally presented.